Electrochemical Oxidation: Understanding the Pathways and Practicalities of PFAS Destruction
June 10, 2024
Author: Louis LeBrun, PE, Axine Water Technologies
As concerns surrounding per- and polyfluoroalkyl substances (PFAS) intensify, the waste management and landfill industries face mounting challenges. Regulatory standards are tightening, necessitating innovative solutions for PFAS destruction. In this context, electrochemical oxidation emerges as a promising method. This article delves into the details of electrochemical oxidation, exploring its mechanisms and practical applications in PFAS remediation.
This is the second of two articles covering the fundamentals of electrochemical destruction of PFAS. To recap the key points of our first article, electrochemical oxidation is a well-proven and cost-effective method for the destruction of PFAS. In its most fundamental description, the process involves applying an electrical current across a pair of reactive electrode elements immersed in the process stream. The actual mechanisms involved in that treatment are the focus of this article.

Electrode and Catalyst Choice Matters
As the name of the process suggests, electrode elements are crucial in the design of any electrochemical treatment process, with a wide range of materials available to choose from. In most cases, the electrode elements consist of a conductive metal core coated with a more highly reactive catalyst material. From lowest to highest reactivity, materials such as tin or lead, precious or rare earth metals (platinum, iridium, etc.), mixed metal oxides, titanium sub-oxides, or boron-doped diamond (BDD) are all options for the treatment process. Each catalyst material determines both the type and rate of chemical reactions available in the treatment process.
Although the inclination might be to choose the most reactive electrode materials, other factors are critical to consider. One of the important factors to consider is the service life and replacement interval of the electrodes, as all catalyst materials have a finite service life and will require regular replacement. While replacement intervals vary based on the application and manufacturer, a service life of two–five years can be expected. Additionally, the electrical efficiency of each material type impacts the operating cost of the system. Balancing service life, replacement cost, and operating cost are crucial for each project. Systems employing a mix of multiple electrode materials usually yield the most effective solution.
Reactions for PFAS Destruction
Electrochemical oxidation is an iteration of advanced oxidation processes (AOPs) increasingly applied in water treatment. The mechanisms of electrochemical oxidation of PFAS involve complex and competitive reaction pathways influenced by factors such as electrode material, electrolyte composition, pH, temperature, applied electrical potential, and reaction kinetics. The key distinction with electrochemical oxidation is its ability to produce high oxidation energy and favorable conditions to promote PFAS destruction.
In practice, PFAS destruction involves successively breaking longer-chain PFAS compounds into short-chain molecules. The complexity of the oxidation process is illustrated in the oxidation destruction pathways for PFOS and PFOA, as shown in Figure 2. At the anode, PFAS molecules undergo oxidative degradation primarily through direct electron transfer reactions. Simultaneously, indirect oxidation occurs using oxidant compounds such as hydroxyl radicals (*OH) generated at the electrode surface. The powerful oxidative conditions created in the electrochemical process allow these reactions to proceed until complete mineralization to elemental precursors are all that remain, such as CO2 gas, H2 gas, fluoride ions, and other mineral salts. Simple pH control is used to prevent the production of hydrogen fluoride (HF) in the destruction process.
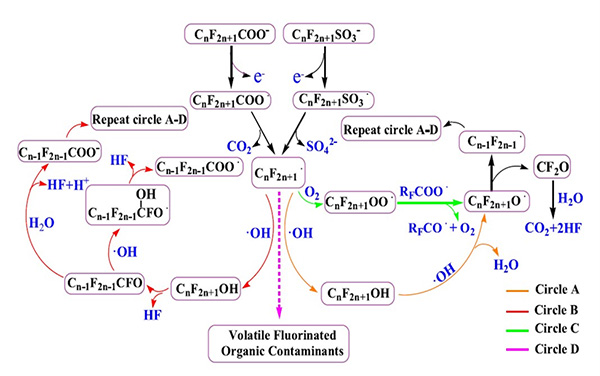
Practical Applications
Research into electrochemical oxidation of PFAS has focused on optimizing reaction conditions, exploring new electrode materials, elucidating reaction mechanisms, and assessing the environmental fate of degradation products. Recent studies have demonstrated the effectiveness of electrochemical oxidation for the treatment of PFAS-contaminated water sources, achieving high removal efficiencies and generating non-toxic byproducts. Challenges remain, including developing cost-effective electrode materials with enhanced catalytic activity, minimizing energy consumption, and mitigating potential risks associated with the formation of transformation by-products.
Evidence that electrochemical oxidation occurs through successive oxidation is easy to see by examining the relative concentration of PFAS compounds at regular intervals throughout the treatment process. Figure 3 shows treatment results for an industrial wastewater sample containing over 26 mg/L of various PFAS compounds and over 43,000 mg/L of COD. Longer-chain PFAS molecules are successively oxidized to produce short chain compounds, continuing until compounds are completely mineralized. In the example shown, over 98.8% destruction of all PFAS compounds was achieved, with removal of most compounds exceeding 99.9%.
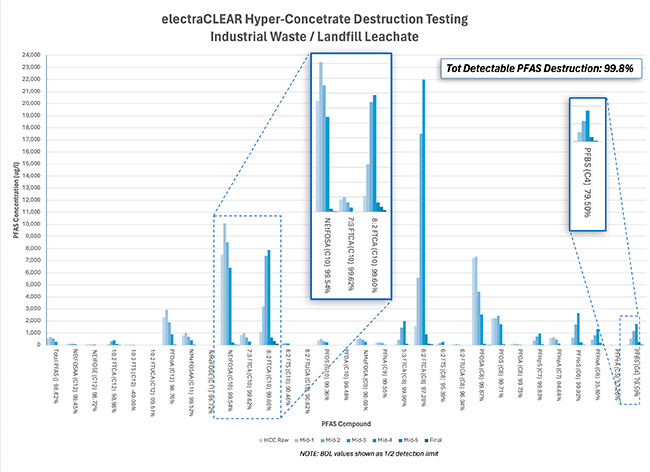
The Importance of Testing
The breadth and scope of PFAS treatment challenges in today’s environment make every application unique. While the oxidation processes described apply to every application, the specific compounds to be treated and water chemistry of every project are unique. To address these issues, lab and pilot-scale testing for each application are critical for project success. Laboratory testing helps the process design team identify the correct mix of electrode and catalyst materials for the most favorable oxidation conditions. Subsequent pilot testing optimizes the overall operating conditions for the treatment process. This 1-2 testing approach ensures that complete PFAS destruction can be achieved cost-effectively.
Offering advantages in efficiency, selectivity, and environmental compatibility, electrochemical oxidation represents one of the most promising approaches for PFAS destruction in contaminated water sources. The powerful oxidizing conditions created and sustained in the process ensure that the full spectrum of PFAS molecules can be destroyed without formation of harmful by-products. Customers taking a systematic approach to evaluating their application can identify the best materials and operating conditions to ensure cost-effective treatment and guaranteed compliance with rapidly evolving regulatory requirements for PFAS.
A Proven Solution
In an era marked by stringent regulatory frameworks and escalating PFAS contamination, the need for effective remediation methods is paramount. Electrochemical oxidation stands at the forefront, offering a blend of efficiency, flexibility, and reliable destruction results. As industries navigate evolving compliance standards, a systematic approach to evaluating electrochemical oxidation can pave the way for cost-effective and sustainable PFAS treatment solutions. Through ongoing research and practical application, electrochemical oxidation continues to redefine the landscape of PFAS remediation, ensuring a cleaner and safer environment for future generations.
About the Author
Louis LeBrun, PE, is a professional engineer with more than 25 years of experience in water and wastewater treatment, including advanced oxidation and electrochemical oxidation processes. During this time, he has been involved in delivering treatment solutions and commercializing treatment technologies throughout North America, South America, Europe, and Asia. In his current role as Vice President of Sales at Axine Water Technologies, he is responsible for the delivery of treatment solutions worldwide. Connect with Louis on LinkedIn, via email at llebrun@axinewater.com, or by phone at (919) 996-9372.
Look out for our future articles on electrochemical PFAS destruction in upcoming issues. For more information about Axine Water Technologies’ electraCLEAR® electrochemical oxidation process, visit www.axinewater.com or call 1 (604) 336-8900.







Leave a comment